Menu
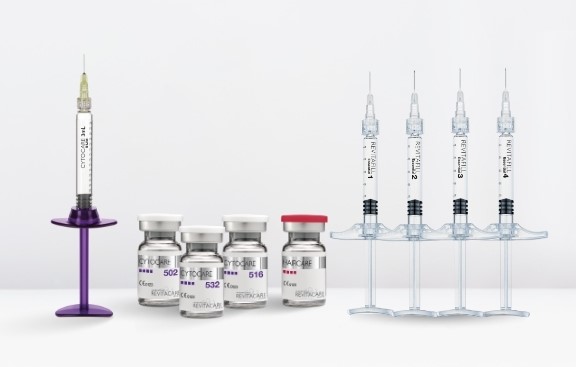
Laboratoire Revitacare has developed different ranges of hyaluronic acid-based of class III Medical Devices. Our Medical Devices comply with the essential requirements of the Council Directive 93/42/ECC concerning Medical Devices and its amendments.
CYTOCARE range is composed by 4 products: CYTOCARE 502-516-532 and CYTOCARE S Line for improving skin quality. CYTOCARE range contains non cross-linked hyaluronic acid with CT50 Rejuvenating complex (vitamins, amino acids, minerals...).
HAIRCARE contains non cross-linked hyaluronic acid and a Restructuring Hair complex (vitamins, minerals,...) for improving scalp and hair quality.
REVITAFILL range is composed by 4 said-biphasic fillers: REVITAFILL Essential 1-2-3-4 contain cross-linked hyaluronic acid dispersed in free hyaluronic acid and different particle sizes. REVITAFILL range is indicated for volume enhancement and correcting facial defects, thanks to 4 fillers with different physicochemical properties.
Our different ranges of Medical Devices are intended for medical doctors.
check_circle
check_circle
Medical Devices products are intended for use by Medical Doctors only, with experience in the anatomy and physiology of the injection site.
Revitacare requires your attention on the fact that none of the information contained in this website should be considered a solicitation, or advertisement for any medical procedure or health product in general.